Expro is professional in meeting the demand for agency service of drug registration with National Medical Products Administration (NMPA). Expro drug registration team can provide customers with regulatory consulting and new drug, generic drug and imported drug registration agency services with NMPA. In the process of registration, our registration team can fully understand and grasp the key points of NMPA’s requirement for registration and the specific requirements for application documents and maintain efficient and effective communication with our customers and NMPA so as to ensure the rapid and smooth progress of the registration project.
The process of registration application shall comply with < Regulations for the Supervision and Administration of Medical Devices> and <Provisions for Medical Device Registration>. NMPA is in charge of reviewing and approving the registration application of medical devices in accordance with the relevant regulations and provisions.
<Regulations for The Supervision and Administration of Medical Devices> (Decree No. 650 of the State Council) came into force on June 1, 2014, and <Provisions for Medical Device Registration> (Decree No. 4 of the State Food and Drug Administration) came into force on October 1, 2014.
The State shall implement classified administration on medical devices based on the degree of risk.
Class I Medical Devices are those with lower degree of risk for which the safety and effectiveness can be ensured through routine management。
Class II Medical Devices are those with medium degree of risk for which further control is required to ensure their safety and effectiveness。
Class III Medical Devices are those with higher degree of risk which must be strictly controlled in respect to safety and effectiveness。
Class I medical device is subject to record administration and class II and class III medical devices are subject to registration administration.
To apply for record of domestic medical devices of class I, the applicant shall submit the documents to the local medical products administration department in the city where the applicant is located.
For domestic medical devices of Class II, they shall be reviewed by medical products administration department of the provinces, autonomous regions and municipalities directly under the central government, and the medical device registration certificate shall be issued after approval.
For domestic medical devices of Class III, they shall be reviewed by the National Medical Products Administration, and the medical device registration certificate shall be issued after approval.
To apply for record of import medical devices of class I, the applicant shall submit the documents to the National Medical Products Administration.
For import medical devices of class II and class III, they shall be reviewed by the National Medical Products Administration, and the medical device registration certificate shall be issued after approval.
The medical devices from Hong Kong, Macao and Taiwan shall be registered and recorded by reference to the imported medical devices.
For record of class I medical devices, the applicant may submit self-testing report on them.
When applying for registration of class II and class III medical devices, registration testing shall be conducted. The medical device testing institutes shall carry out relevant registration testing on them in accordance with their technical requirements.

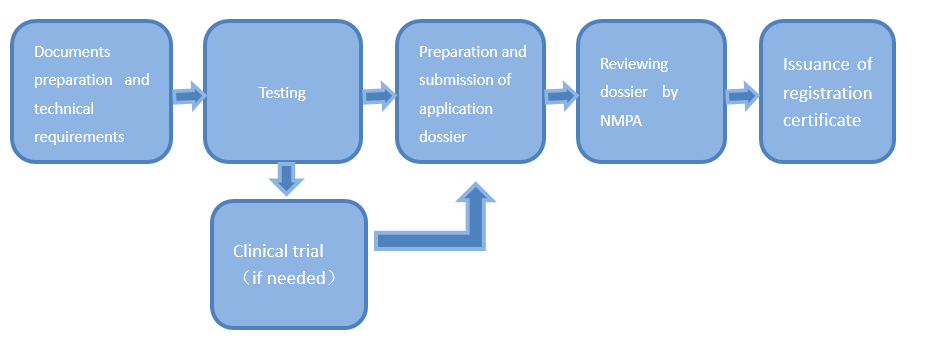
Medical device registration refers to the prescribed procedures conducted by medical products administration department upon an application dossier submitted by the registration applicant to decide whether the medical device to be marketed can be sold based on a comprehensive evaluation of the research and results of its safety and effectiveness.