Expro is professional in meeting the demand for agency service of drug registration with National Medical Products Administration (NMPA). Expro drug registration team can provide customers with regulatory consulting and new drug, generic drug and imported drug registration agency services with NMPA. In the process of registration, our registration team can fully understand and grasp the key points of NMPA’s requirement for registration and the specific requirements for application documents and maintain efficient and effective communication with our customers and NMPA so as to ensure the rapid and smooth progress of the registration project.

Expro is an expert in satisfying the demand for agency service of medical device registration with China National Medical Products Administration (NMPA). Our medical device registration team provides our customers with professional medical device registration agency services, including drafting technical requirements, product registration testing, preparation and submission of registration application dossier and the follow-up of the whole registration process.
In order to regularize their registration and register management and ensure their safety and effectiveness, the medical devices, no matter where they are produced, need to apply for Medical Device Registration Certificate issued by NMPA before they can be sold in Chinese market.
The process of registration application shall comply with < Regulations for the Supervision and Administration of Medical Devices> and <Provisions for Medical Device Registration>. NMPA is in charge of reviewing and approving the registration application of medical devices in accordance with the relevant regulations and provisions.
<Regulations for The Supervision and Administration of Medical Devices> (Decree No. 650 of the State Council) came into force on June 1, 2014, and <Provisions for Medical Device Registration> (Decree No. 4 of the State Food and Drug Administration) came into force on October 1, 2014.

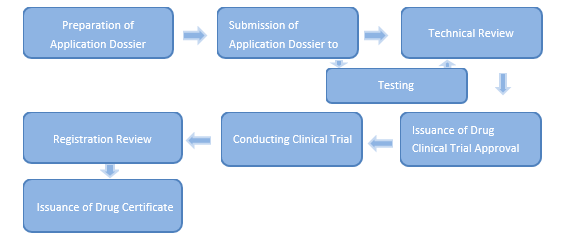
I. Clinical Trial of New Drug
1. After completing the pre-clinical study, the applicant shall fill the Application Form for Drug Registration, and report authentically relevant materials to the drug regulatory department of the province, autonomous region or municipality directly under the Central Government where the applicant is located.
2. Drug regulatory departments of provinces, autonomous regions, or municipalities directly under the Central Government shall conduct the preliminary review of the application dossiers, and issue a acceptance notice of drug registration application if requirements are met, or issue a non-acceptance notice, in which reasons shall be given, of drug registration application if requirements are not met.
3. Drug regulatory departments of provinces, autonomous regions, or municipalities directly under the Central Government shall organize to conduct on-site inspections of the drug research and development conditions and raw data, make preliminary review of the submitted dossiers, and provide review opinions within five days from the date it accepts an application. Where the drug for which the registration is applied is a biological product, samples from three production batches thereof shall also be collected for testing, and a notice for the testing for registration shall be issued to the drug testing institute.
4. Drug regulatory departments of provinces, autonomous regions, or municipalities directly under the Central Government shall deliver the review opinions, inspection reports and the application dossiers to the Center for Drug Evaluation of the National Medical Products Administration within the specified timeline, and notice the applicants.
5. The drug testing institute that receives a notice for the testing for registration shall test the samples according to the drug specifications submitted by the applicant, verify the submitted drug specifications, and submit a certificate of analysis for drug registration to the Center for Drug Evaluation of the National Medical Products Administration within the specified timeline, and copy to the applicant.
6. After receiving submitted dossiers, the Center for Drug Evaluation of the National Medical Products Administration shall organize pharmaceutical, medical and other technical personnel to conduct technical review of the submitted dossiers within the specified timeline, and may request, with reasons, applicants to provide supplementary materials when necessary. After completing technical reviews, the Center for Drug Evaluation shall give technical review opinions and report together with relevant documents to the National Medical Products Administration.
The National Medical Products Administration shall make review and approval decisions based on the technical review opinions. Where the regulations are conformed to, a Drug Clinical Trial Approval shall be issued and where the regulations are not conformed to, a Disapproval Notice shall be issued with reasons provided.
The regular timeline of reviewing clinical trial application is 90 days. For a drug which is entitled to special reviewing procedure, the timeline of reviewing its clinical trial application is 80 days.
II Application for New Drug Registration
1. After completing drug clinical trials, applicants shall fill the Application Form for Drug Registration, submit production application dossiers to the drug regulatory departments of the provinces, autonomous regions, or municipalities directly under the Central Government where the applicant is located, and at the same time provide the raw materials and research information for preparing reference standards to the National Institute for the Control of Pharmaceutical and Biological Products.
2. Drug regulatory departments of provinces, autonomous regions, or municipalities directly under the Central Government shall conduct the preliminary review of the application dossiers, and issue an acceptance notice of drug registration application if requirements are met, or issue a non-acceptance notice, in which reasons shall be given, of drug registration application if requirements are not met.
3. Drug regulatory departments of provinces, autonomous regions, or municipalities directly under the Central Government shall organize to conduct on-site inspections of the drug research and development conditions and raw data, make preliminary review of the submitted dossiers, and provide review opinions within five days from the date it accepts an application. For the other drugs except biological products, samples of three production batches shall also be collected for testing, and a notice of specifications verification shall be issued to the drug testing institute.
4. Drug regulatory departments of provinces, autonomous regions, or municipalities directly under the Central Government shall deliver the review opinions, inspection reports and the application dossiers to the Center for Drug Evaluation of the National Medical Products Administration within the specified timeline, and notice the applicants.
5. Drug testing institutes shall verify the submitted drug specifications and give the verification opinions to the Center for Drug Evaluation of the National Medical Products Administration within the specified timeline, and at the same time copy to the drug regulatory departments of the provinces, autonomous regions, or municipalities directly under the Central Government that notify them to conduct the verification, and the applicants.
6. After receiving submitted dossiers, the Center for Drug Evaluation of the National Medical Products Administration shall organize pharmaceutical, medical and other technical personnel to conduct technical review of the submitted dossiers within the specified timeline, and may request, with reasons, applicants to provide supplementary materials when necessary.
Where the regulations are conformed to as reviewed, the Center for Drug Evaluation of the National Medical Products Administration shall notice the applicant to apply for production site inspection and inform the Center for Drug Certification of the National Medical Products Administration. Where the regulations are not conformed to as reviewed, the Center for Drug Evaluation of the National Medical Products Administration shall report the review opinions and relevant documents to the National Medical Products Administration; the National Medical Products Administration shall make a disapproval decision to the application based on the technical review opinions, and issue a Disapproval Notice with reasons.
7. The applicant shall apply for on-site inspection to the Center for Drug Certification of the National Medical Products Administration within six months from the date it receives the notice of production site inspection.
8. The Center for Drug Certification of the National Medical Products Administration shall, within 30 days from the date it receives the application for production site inspection, organize on-site inspection of large-scale samples production, verify the applicability of the manufacturing processes and at the same time take samples of one batch of products (samples of three batches of products for biological products), and provide production site inspection report to the Center for Drug Evaluation of the National Medical Products Administration within ten days after the site inspection.
9. Drug testing institutes shall conduct sample testing according to the verified specifications, and within the specified timeline, provide testing reports to the Center for Drug Evaluation of the National Medical Products Administration, and copy to the relevant drug regulatory departments of the provinces, autonomous regions, or municipalities directly under the Central Government and the applicants.
10. The Center for Drug Evaluation of the National Medical Products Administration shall make a general opinion based on the technical review opinions, production site inspection reports and sample testing results, and report the general opinion together with relevant documents to the National Medical Products Administration. The National Medical Products Administration shall make a review and approval decision based on the general opinion. Where the regulations are conformed to, a New Drug Certificate shall be issued; if the applicant already has a Drug Manufacturing Certificate and possesses the production conditions, a drug approval number shall be issued at the same time; where the regulations are not conformed to, a Disapproval Notice shall be issued with reasons.
The regular timeline of reviewing registration application is 150 days. For a drug which is entitled to special reviewing procedure, the timeline of reviewing its registration application is 120 days.

A drug being applied for importation shall have already obtained the drug marketing authorization in the producing country or region where the overseas pharmaceutical manufacturer is located; those not yet obtained marketing authorization in the producing country or region, however confirmed with safety, efficacy and clinical needs by the National Medical Products Administration may be approved for importation.
The production of a drug applied for importation shall comply with the GMP requirements of both the producing country or region where the drug manufacturer is located and China.
1. To apply for import drug registration, the applicant shall fill the Application Form for Drug Registration, submit relevant dossiers and samples, provide relevant approval documents, and submit the application to the National Medical Products Administration.
2. National Medical Products Administration shall conduct the preliminary review of the application dossiers, and issue an acceptance notice of drug registration application and notify the National Institute for the Control of Pharmaceutical and Biological Products to conduct testing for registration of samples from three batches if requirements are met; or issue a nonacceptance notice of drug registration application with reasons if requirements are not met.
National Medical Products Administration may organize to conduct on-site inspection of development and production conditions and take samples.
3. The National Institute for the Control of Pharmaceutical and Biological Products shall organize to conduct the testing for drug registration within five days from the date it receives the dossiers and samples.
4. The drug testing institutes undertaking the import drug testing shall complete the testing for registration and submit the certificate of analysis for drug registration to the National Institute for the Control of Pharmaceutical and Biological Products within 60 days from the date they receive the documents, samples and relevant reference standards. Sample testing and verification of specifications for controlled drugs or vaccines shall be completed within 90 days.
5. The National Institute for the Control of Pharmaceutical and Biological Products shall organize experts to conduct technical review within 20 days from the date it receives the certificate of analysis for drug registration and the verified import specifications, and if necessary, conduct further verification according to the review opinions.
6. After completing the testing for import drug registration, the National Institute for the Control of Pharmaceutical and Biological Products shall give the verified specifications, certificate of analysis and opinions thereof to the Center for Drug Evaluation of the National Medical Products Administration, and copy the applicants.
7. The Center for Drug Evaluation of the National Medical Products Administration shall organize pharmaceutical, medical and other technical personnel to conduct technical review of the submitted dossiers within the specified timeline, and may request, with reasons, applicants to provide supplementary materials when necessary.
8. The Center for Drug Evaluation of the National Medical Products Administration shall make a general opinion based on the technical review opinions and sample testing results and report the general opinion together with relevant documents to the State Food and Drug Administration. The State Food and Drug Administration shall make a review and approval decision based on the general opinion. Where the regulations are conformed to, a Clinical Trial Approval shall be issued; where the regulations are not conformed to, a Disapproval Notice shall be issued with reasons.
9. After a clinical trial application is approved, the applicant shall conduct the trial in accordance with the requirements in Provisions for Drug Registration and the other relevant requirements.
After a clinical trial is completed, the applicant shall fill the Application Form for Drug Registration, submit the clinical trial data, other altered and supplementary data in accordance with regulations, give in detail the basis and reasons, and provide relevant approved documents.
10. The Center for Drug Evaluation of the National Medical Products Administration shall organize pharmaceutical, medical and other technical personnel to conduct comprehensive review of the submitted clinical trial data within the specified timeline, and may request, with reasons, applicants to provide supplementary materials when necessary.
11. The National Medical Products Administration shall make a review and approval decision based on the general opinion. An Import Drug License shall be issued if regulations are conformed to. For a drug applied for registration by a drug manufacturer in Hong Kong, Macao or Taiwan of China, its application shall be handled by reference to the application procedures for import drug registration. If requirements are met, a Pharmaceutical Product License shall be issued; if requirements are not met, a Disapproval Notice shall be issued with reasons.